Lithium-ion batteries (LIBs) have become the standard for electrochemical energy storage in consumer electronics and electric vehicles because of their many desirable qualities, including high energy density, high power density, and long cycle life. Although energy storage capacity, cycle life, and cost are of primary importance for LIBs, there is growing interest and concern regarding the overall life cycle environmental impacts of LIBs. This topic has become increasingly important due to the rapid growth of the worldwide LIB market and the accompanying surge in LIB materials extraction, production, and disposal.
Life cycle assessment (LCA) is a powerful methodology that seeks to quantify the environmental impacts of a product’s materials, manufacturing, use, and disposal across a range of impact categories. LIB LCA studies to-date have predominantly followed a “traditional” LCA approach focused on comparing the environmental impacts of commercially-available batteries. In this work, we are undertaking for the first time the integration of battery LCA and first-principles electrochemical modeling to study the effects of cell design and operation conditions on LIB life cycle environmental impacts. Research studies conducted to-date explore the effect of electrode thickness, electrode porosity, discharge rate, ambient temperature, and forced-air cooling on the life cycle environmental impacts of lithium iron phosphate-graphite (LFP-C) and lithium cobalt oxide-graphite (LCO-C) batteries.
Highlights:
- First-principles electrochemical modeling is integrated with battery life cycle assessment for the first time.
- Specific values of electrode thickness and porosity that minimize lithium-ion battery environmental impacts are determined..
- Optimum cell designs for minimizing lithium-ion battery life cycle environmental impacts are considered for varying discharge rates and ambient temperatures.
- Exploration of numerous environmental impact categories (including: global warming potential, mineral depletion potential, energy usage, PM2.5, PM10, sulfur oxides, and nitrogen oxides) for two different lithium-ion battery chemistries (LFP-C, LCO-C).
Publications:
- Douglas Pedersen, Michael Lybbert, and Roseanne Warren, “Life cycle analysis of LiCoO2/graphite batteries with cooling using combined electrochemical-thermal modeling,” Resources, Conservation & Recycling, Vol. 180, pp. 106204, 2022. link
- Michael Lybbert, Zahra Ghaemi, A.K. Balaji, and Roseanne Warren, “Integrating life cycle assessment and electrochemical modeling to study the effects of cell design and operating conditions on the environmental impacts of lithium-ion batteries,” Renewable & Sustainable Energy Reviews, Vol. 144, pp. 111004, 2021. link
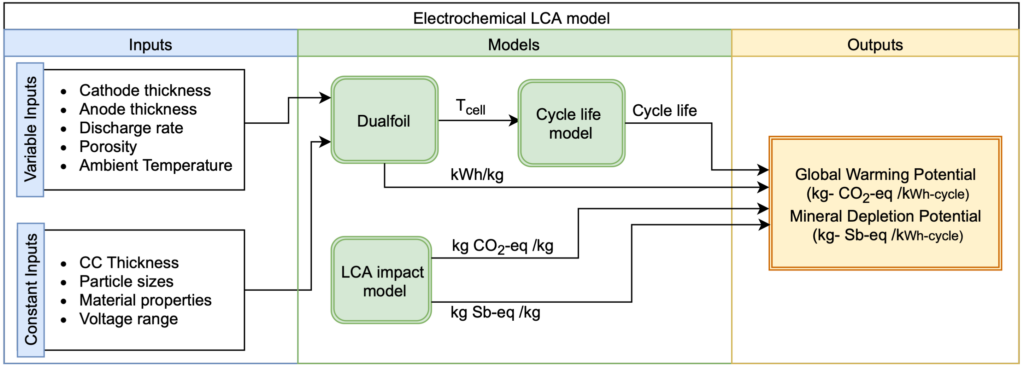
In our electrochemical LCA model, battery design parameters and operating conditions are inputs to the electrochemical model (Dualfoil), which calculates cell temperature (an input to the cycle life model) and energy density. Cycle life and energy density values are combined with kg CO2-eq/kg and kg Sb-eq/kg values from the LCA impact model to determine GWP and MDP per kWh and per kWh-cycle.
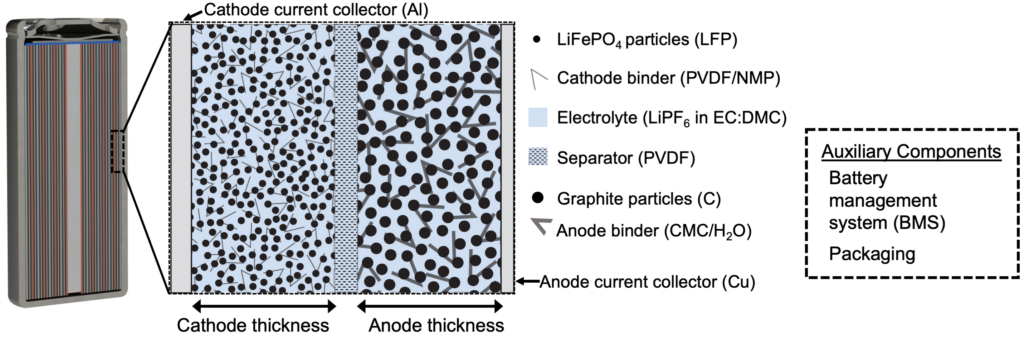
Conceptual illustration of an LFP-C battery under study in our electrochemical LCA research.
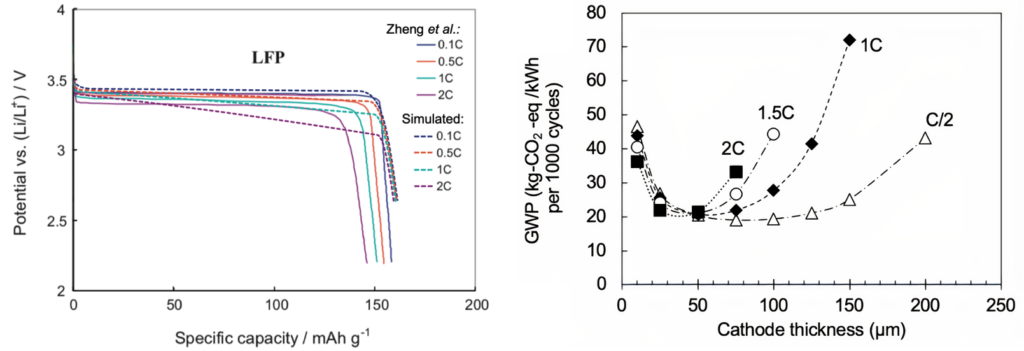
Dualfoil model validation vs. Zheng et al. Electrochim Acta 2012;71:258-65 (Left).
Impact of cell discharge rate and cathode thickness on the global warming potential (GWP) of LFP-C batteries (discharge rates given as C-rates) (Right).
Funding: This work was supported by The Global Change & Sustainability Center at the University of Utah through the GCSC First Year Fellowship program.