Supercapacitors are electrochemical energy storage devices with high power density and long cycle life compared to batteries. Supercapacitors store charge in the form of electric double layer capacitance or by additional surface or near-surface Faradaic reactions by means of pseudocapacitance. The Advanced Energy Innovation’s lab current research on supercapacitors is focused on studies of Utah coal char-derived carbons as an extremely low-cost electrode material for electrochemical double layer capacitors. PI Warren has also made substantial contributions to the development of ultra high-energy density pseudocapacitors via atomic layer deposition of redox active thin films on vertically aligned carbon nanotubes (CNTs).
Vertically-Aligned Carbon Nanotube Pseudocapacitors via Atomic Layer Deposition
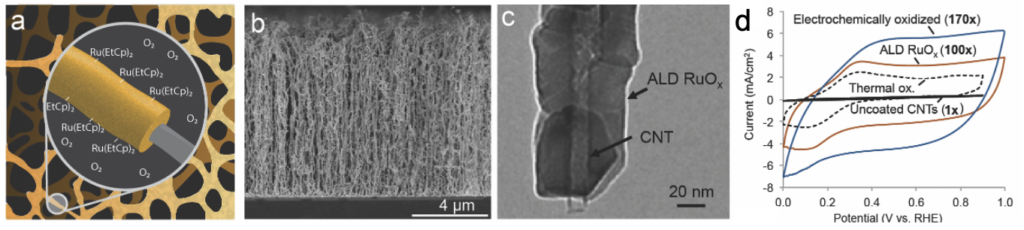
Large surface area electrodes and highly active pseudocapacitive materials are paramount for achieving high performance supercapacitors. To achieve high specific capacitance, pseudocapacitor nanoscale fabrication methods that provide excellent control of electrode structure and chemical composition as well as good uniformity over high surface areas are highly beneficial. Atomic layer deposition (ALD) is a high-precision chemical vapor deposition technique that uses sequential pulsing of precursors to deposit nanoscale films monolayer by monolayer. With ALD, conformal and uniform films of metal, oxide, and nitride materials can be deposited onto high surface area substrates. PI Warren’s contributions in the field of ALD pseudocapacitors include: the first demonstration of ALD RuOx coatings for pseudocapacitor applications, the first investigations of the effects of ALD process conditions on pseudocapacitor thin-film performance based on level of in-situ ALD oxidation and hydration, and the first demonstrations of ALD TiS2 and TiN pseudocapacitors.
Utah Coal Char-Derived Electrochemical Double Layer Capacitors
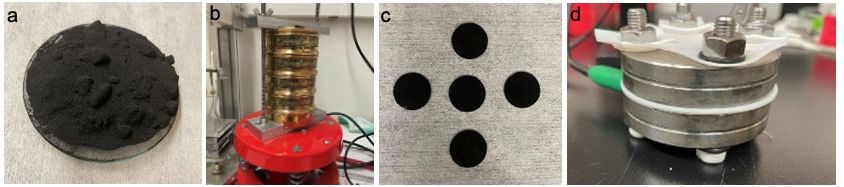
Utah coal char is an ultra-low cost carbon source with promising applications in energy storage, including as an electrode material for electrochemical double layer capacitors. The cost of coal char is approximately $0.01-$0.03/kg, compared with the value of commercial carbon battery and supercapacitor anodes at approximately $12.50/kg. Char is a constituent of lignite, sub-bituminous, and bituminous-type coals, and is a complex mixture of ash, hydrocarbons, and hard carbons. While coal tar (liquid component of pyrolyzed coals) is a high-value material that has been used commercially as an anode material for lithium-ion batteries, current applications of coal char are largely limited to energy production by combustion. In this work we are exploring the effects of processing conditions and electrolyte composition on the supercapacitor performance of Utah coal char. Results to-date are highly promising, with untreated coal char achieving energy densities 1/3 that of graphene electrodes when tested in 12.5 M NaClO4 water-in-salt electrolyte.
Highlights
- ALD RuOx demonstrated as high-performance pseudocapacitive thin film, especially at low ALD temperatures and with post-ALD electrochemical oxidation to produce a hydrated oxide.
- TiS2–VACNT composite electrodes demonstrated with a large electrochemical voltage window of 3 V and energy density of 61 Wh/kg.
- Utah coal char is found to be a competitive, ultra low-cost material for electrochemical double layer capacitors, achieving 5.8 Wh/kg in water-in-salt NaClO4 electrolyte.
Publications, Presentations & Patent:
- Zahra Karimi, Jaron Moon, Chanel Van Ginkel, Douglas Pedersen, Joshua Malzahn, Eric Eddings, and Roseanne Warren, “Effect of electrolyte composition on the performance of coal char-derived carbon supercapacitors,” Proceedings of the 239th ECS Meeting, Online meeting, May 2021 (Oral presentation).
- Xining Zang, Caiwei Shen, Emmeline Kao, Roseanne Warren, Ruopeng Zhang, Kwok Siong Teh, Junwen Zhong, Minsong Wei, Buxuan Li, Yao Chu, Mohan Sanghadasa, Adam Schwartzberg, and Liwei Lin, “Titanium Disulfide Coated Carbon Nanotube Hybrid Electrodes Enable High Energy Density Symmetric Pseudocapacitors,” Advanced Materials, Vol. 30, pp. 1704754, 2018. DOI: 10.1002/adma.201704754.
- Emmeline Kao, Chen Yang, Roseanne Warren, Alina Kozinda, and Liwei Lin, “ALD Titanium Nitride on Vertically Aligned Carbon Nanotube Forests for Electrochemical Supercapacitors,” Sensors and Actuators A: Physical, Vol. 240, pp. 160-166, 2016. DOI: 10.1015/j.sna.2016.01.044.
- Roseanne Warren, Firas Sammoura, Fares Tounsi, Mohan Sanghadasa and Liwei Lin, “Highly Active Ruthenium Oxide Coating via ALD and Electrochemical Activation in Supercapacitor Applications,” Journal of Materials Chemistry A, Vol. 3, pp. 15568-15575, 2015. DOI: 10.1039/C5TA03742E.
- Roseanne Warren, Firas Sammoura, Kwok Siong Teh, Alina Kozinda, Xining Zang, and Liwei Lin “Electrochemically Synthesized and Vertically Aligned Carbon Nanotube-Polypyrrole Nanolayers for High Energy Storage Devices,” Sensors and Actuators – A Physical, Vol. 231, pp. 65-73, 2015. DOI: 10.1016/j.sna.2014.07.010.
- Roseanne Warren, Firas Sammoura, and Liwei Lin, “Fabrication of enhanced supercapacitors using atomic layer deposition of metal oxide on nanostructures” US Patent No. 9805880.
Funding: NSF Award# 1742696
a) Conceptual illustration of ALD RuOx deposition on high surface area, porous materials using Ru(EtCp)2 and O2 as precursors. b) SEM image of VACNTs coated with ALD RuOx. c) TEM image of a single CNT coated with ALD RuOx. d) Cyclic voltammetry measurements indicating significant improvements in the capacitance of VACNT electrodes coated with thermally-oxidized ALD RuOx vs. as-deposited ALD RuOx vs. electrochemically oxidized ALD RuOx.
Preparation and testing of Utah coal-char derived electrochemical double layer capacitors. a) Coal char powder following pyrolyzation at 900 deg C. b) Sieving and grinding of the coal char to achieve the desired particle size (< 53 µm). c) Coal char electrodes prepared on stainless steel current collectors. d) Supercapacitor testing using a symmetrical two-electrode Conflat cell configuration.